Given That The Molar Mass Of Nacl Is 58.44
New Snow
May 10, 2025 · 6 min read
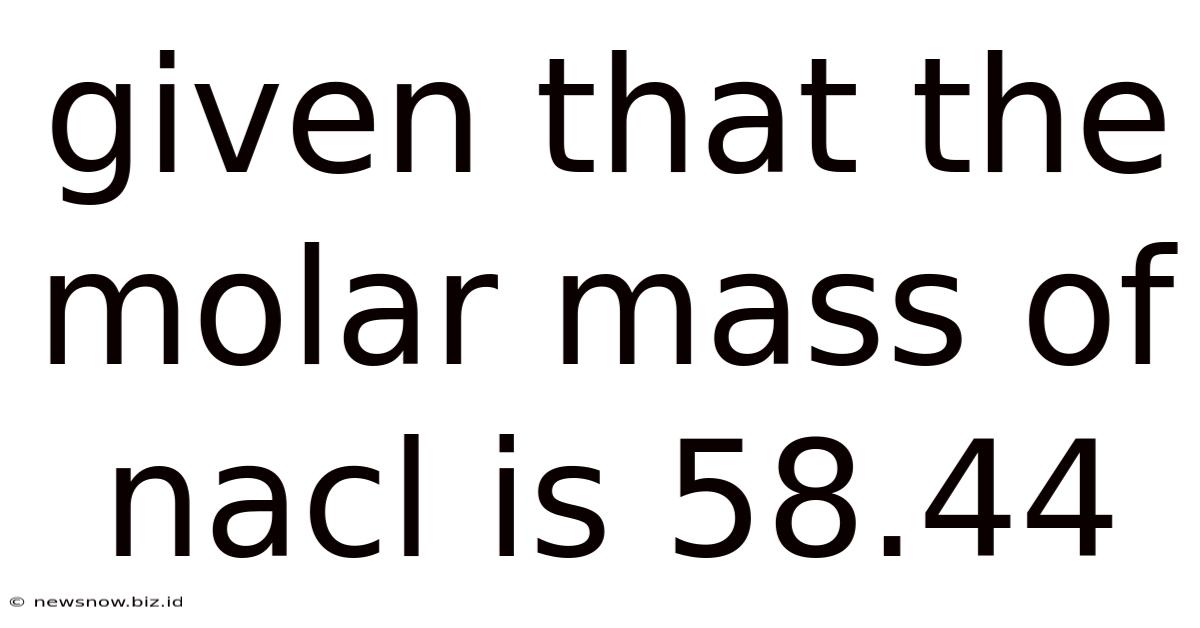
Table of Contents
Delving Deep into NaCl: Exploring Properties and Applications Given its Molar Mass of 58.44 g/mol
The seemingly simple compound, sodium chloride (NaCl), commonly known as table salt, holds a world of fascinating properties and applications far beyond its culinary uses. Knowing its molar mass of 58.44 g/mol is a crucial starting point for understanding its behavior in various chemical and physical contexts. This article will delve into the diverse aspects of NaCl, exploring its properties, applications, and significance across various fields, all while leveraging the knowledge of its molar mass.
Understanding the Significance of Molar Mass (58.44 g/mol)
The molar mass of NaCl, 58.44 g/mol, represents the mass of one mole of NaCl molecules. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of entities, in this case, NaCl molecules. This value is calculated by summing the atomic masses of its constituent elements: sodium (Na) with an atomic mass of approximately 22.99 g/mol and chlorine (Cl) with an atomic mass of approximately 35.45 g/mol. Therefore, 22.99 g/mol + 35.45 g/mol = 58.44 g/mol.
This seemingly simple figure is crucial for various calculations:
-
Stoichiometry: Molar mass is essential for calculating the amounts of reactants and products in chemical reactions involving NaCl. For example, it allows us to determine the mass of NaCl needed to react completely with a specific amount of another substance.
-
Solution Preparation: In preparing solutions of a specific concentration (e.g., molarity), the molar mass is used to calculate the mass of NaCl required to dissolve in a given volume of solvent.
-
Determination of Empirical and Molecular Formulas: Molar mass is an integral part of determining the empirical and molecular formulas of unknown compounds through experimental data.
-
Thermodynamic Calculations: Molar mass is utilized in various thermodynamic calculations, such as determining enthalpy changes, entropy changes, and Gibbs free energy changes in reactions involving NaCl.
Physical and Chemical Properties of NaCl
Knowing the molar mass provides a foundation for understanding NaCl's properties:
Physical Properties:
-
Crystalline Structure: NaCl exists as a crystalline solid with a cubic close-packed structure. This arrangement contributes to its characteristic properties, including its brittleness and high melting point. The strong electrostatic forces between the positively charged sodium ions (Na⁺) and the negatively charged chloride ions (Cl⁻) are directly linked to its molar mass and the strength of the ionic bonds.
-
Solubility: NaCl is highly soluble in water due to the strong ion-dipole interactions between the polar water molecules and the charged ions. The dissolution process involves the separation of Na⁺ and Cl⁻ ions, which are then surrounded by water molecules, forming hydrated ions. The molar mass influences the concentration of ions in solution once a certain mass is dissolved.
-
Melting and Boiling Points: NaCl has a relatively high melting point (801 °C) and boiling point (1413 °C) due to the strong electrostatic attraction between its ions. The energy required to overcome these attractions and transition to the liquid or gaseous phase is directly related to its molar mass and the strength of the ionic bonds.
-
Density: NaCl has a density of approximately 2.16 g/cm³. This density is influenced by the arrangement of ions in its crystalline structure, linked again to its molar mass and the ionic interactions.
Chemical Properties:
-
Ionic Nature: NaCl is an ionic compound, formed by the electrostatic attraction between Na⁺ and Cl⁻ ions. This ionic nature governs its behavior in various chemical reactions.
-
Reactivity: While relatively unreactive, NaCl can participate in certain reactions. For example, it can react with sulfuric acid (H₂SO₄) to produce hydrogen chloride (HCl) gas. The molar mass is important in determining the stoichiometry of such reactions.
-
Electrolysis: NaCl undergoes electrolysis, a process where an electric current is passed through a molten or aqueous solution to produce sodium metal (Na) and chlorine gas (Cl₂). Understanding the molar mass helps calculate the amount of sodium and chlorine produced in the process.
Diverse Applications of NaCl
The versatility of NaCl is evident in its wide range of applications:
Food Industry:
-
Flavor Enhancer: NaCl is widely used as a flavor enhancer and preservative in food products. Its taste and ability to inhibit microbial growth are crucial to the food industry.
-
Food Preservation: NaCl is a natural preservative, preventing microbial spoilage in many foods. The molar mass is not directly involved in this application but is a fundamental part of understanding the chemical properties underpinning this use.
Chemical Industry:
-
Production of Chlorine and Sodium Hydroxide: The electrolysis of NaCl is a crucial industrial process for the production of chlorine and sodium hydroxide, which are essential components in many chemical products. The molar mass is central to calculating yields and efficiencies in this process.
-
Production of other Sodium Compounds: NaCl serves as a raw material for the production of numerous sodium compounds, such as sodium carbonate (Na₂CO₃) and sodium sulfate (Na₂SO₄). Again, stoichiometric calculations utilizing molar mass are crucial here.
Medical Applications:
-
Electrolyte Solution: NaCl is a crucial component in intravenous (IV) solutions, providing essential electrolytes for patients. The precise calculation of concentration based on molar mass ensures the safety and efficacy of these solutions.
-
Wound Cleaning: NaCl solutions are used to clean wounds due to their ability to dissolve and remove impurities.
Other Applications:
-
De-icing Agent: NaCl is frequently used as a de-icing agent for roads and pavements during winter.
-
Water Softening: NaCl is used in water softening processes to regenerate ion-exchange resins.
-
Textile Industry: NaCl plays a role in certain dyeing and bleaching processes in the textile industry.
Environmental Considerations
While essential, the excessive use of NaCl can have environmental implications:
-
Water Pollution: Runoff from roads treated with NaCl can contribute to water pollution, impacting aquatic ecosystems.
-
Soil Salinity: Excessive use of NaCl in agriculture can lead to soil salinization, impairing plant growth.
Therefore, responsible and sustainable use of NaCl is essential to minimize its environmental impact.
Conclusion: NaCl – A Compound of Profound Importance
The molar mass of NaCl (58.44 g/mol) serves as a fundamental building block for understanding this seemingly simple yet incredibly versatile compound. Its role extends far beyond the kitchen table, impacting various industries and aspects of our daily lives. From food preservation to industrial chemical processes and medical applications, NaCl's significance is undeniable. However, it's crucial to consider its environmental implications and advocate for its responsible and sustainable use. Understanding its properties and applications, underpinned by the knowledge of its molar mass, allows us to appreciate the profound impact of this ubiquitous compound. Further research into the potential of NaCl in emerging fields like nanotechnology and advanced materials science promises even greater discoveries in the future. Its simple formula belies a complex and crucial role in our world.
Latest Posts
Related Post
Thank you for visiting our website which covers about Given That The Molar Mass Of Nacl Is 58.44 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.