Each Hemoglobin Molecule Can Transport Two Molecules Of Oxygen
New Snow
May 11, 2025 · 5 min read
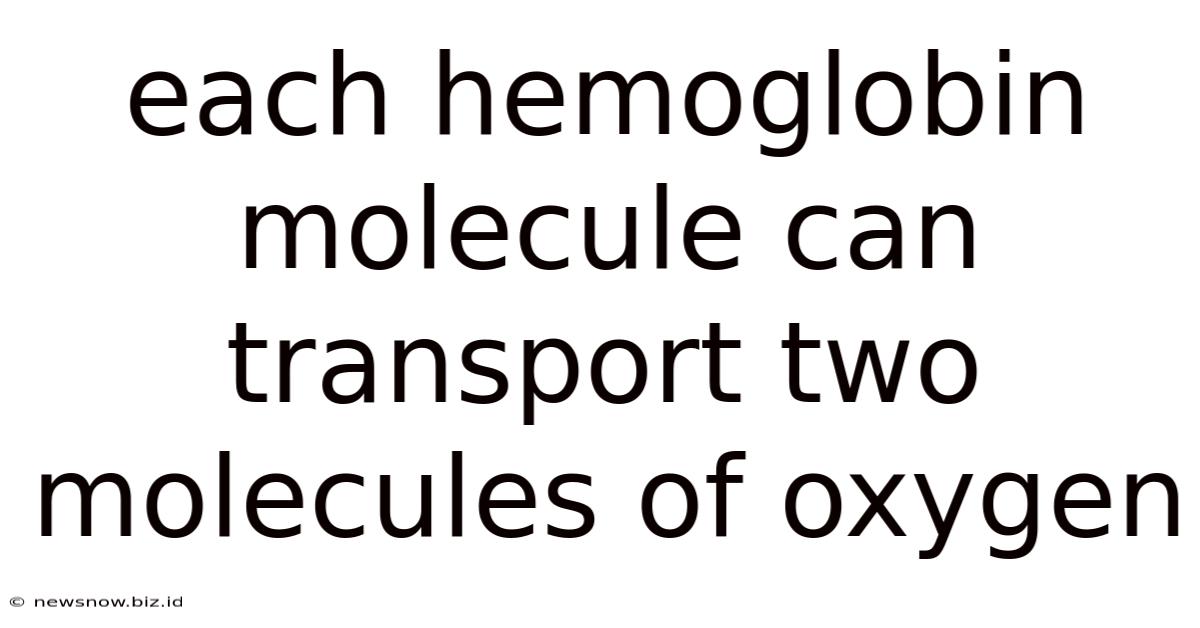
Table of Contents
Debunking the Myth: Each Hemoglobin Molecule Can Transport Four, Not Two, Oxygen Molecules
The statement "each hemoglobin molecule can transport two molecules of oxygen" is fundamentally incorrect. A single hemoglobin molecule, the protein responsible for oxygen transport in red blood cells, can actually bind and carry four molecules of oxygen. This crucial fact underpins our understanding of respiration, oxygen delivery, and overall human physiology. Let's delve into the details of hemoglobin's structure and function to clarify this misconception and explore the complexities of oxygen transport.
Understanding Hemoglobin's Structure: The Key to Oxygen Binding
Hemoglobin is a remarkable protein composed of four subunits, each containing a heme group. This quaternary structure is essential for its oxygen-carrying capacity. The four subunits are arranged in a tetrahedral configuration, allowing for cooperative binding of oxygen molecules.
-
Subunits: Each subunit is a globular protein, with two alpha (α) and two beta (β) chains in adult hemoglobin (HbA). Fetal hemoglobin (HbF) has slightly different subunits (two alpha and two gamma (γ) chains), which exhibit a higher affinity for oxygen, facilitating oxygen transfer from mother to fetus.
-
Heme Group: The heart of each subunit is the heme group, a porphyrin ring complex containing a central iron (Fe) ion. This iron ion is crucial for oxygen binding. The iron exists in the ferrous state (Fe²⁺), which can reversibly bind to an oxygen molecule.
The Cooperative Binding of Oxygen: A Positive Feedback Loop
The binding of oxygen to one heme group doesn't occur independently; it influences the binding affinity of the other heme groups. This phenomenon is known as cooperative binding, and it's crucial for efficient oxygen uptake in the lungs and release in the tissues.
-
Initial Binding: The initial binding of an oxygen molecule to one of the heme groups induces a conformational change in the entire hemoglobin molecule. This change makes it easier for subsequent oxygen molecules to bind to the remaining heme groups.
-
Increased Affinity: As more oxygen molecules bind, the affinity of hemoglobin for oxygen increases, leading to a sigmoidal oxygen-hemoglobin dissociation curve. This curve reflects the cooperative nature of oxygen binding.
-
Oxygen Release: In tissues with low oxygen partial pressure (pO₂), the reverse process occurs. The release of one oxygen molecule decreases the affinity of hemoglobin for the remaining oxygen molecules, facilitating efficient oxygen unloading in tissues requiring it.
Factors Affecting Hemoglobin's Oxygen Binding Affinity
Several factors modulate hemoglobin's affinity for oxygen, influencing the efficiency of oxygen transport. These include:
-
pH: A decrease in pH (increased acidity) reduces hemoglobin's affinity for oxygen, the Bohr effect. This is particularly important in actively metabolizing tissues, where increased carbon dioxide production lowers pH, promoting oxygen release.
-
Carbon Dioxide (CO₂): High CO₂ levels also decrease hemoglobin's affinity for oxygen, contributing to the Bohr effect. CO₂ can bind directly to hemoglobin, forming carbaminohemoglobin.
-
2,3-Bisphosphoglycerate (2,3-BPG): This molecule, present in red blood cells, binds to hemoglobin, reducing its affinity for oxygen. 2,3-BPG levels can be regulated to adjust oxygen delivery based on physiological needs.
-
Temperature: Increased temperature decreases hemoglobin's oxygen affinity, promoting oxygen release in metabolically active tissues that generate heat.
The Significance of Four Oxygen Molecules Per Hemoglobin: Efficient Oxygen Transport
The ability of each hemoglobin molecule to bind four oxygen molecules is essential for efficient oxygen transport throughout the body. If each molecule could only carry two, the oxygen-carrying capacity of blood would be drastically reduced. This would severely impair the body's ability to supply oxygen to tissues, leading to hypoxia and potentially fatal consequences.
-
Increased Carrying Capacity: The four oxygen-binding sites maximize the amount of oxygen transported per hemoglobin molecule. This is crucial considering the relatively low solubility of oxygen in blood plasma.
-
Efficient Delivery to Tissues: The cooperative binding and factors modulating oxygen affinity ensure efficient oxygen loading in the lungs and unloading in the tissues. This precise regulation is vital for meeting the varying oxygen demands of different tissues.
Clinical Implications of Hemoglobin Function
Understanding the intricacies of hemoglobin's structure and function has significant clinical implications. Numerous diseases affect hemoglobin's ability to bind and transport oxygen effectively:
-
Anemia: Characterized by reduced red blood cell count or hemoglobin concentration, anemia leads to impaired oxygen delivery to tissues. Various types of anemia exist, each with different causes and mechanisms.
-
Sickle Cell Anemia: A genetic disorder causing a change in the beta-globin chain, resulting in abnormal hemoglobin (HbS) that polymerizes, leading to deformed red blood cells. These sickle-shaped cells impede blood flow and oxygen delivery.
-
Thalassemia: A group of inherited blood disorders affecting hemoglobin production. Different types of thalassemia affect the synthesis of alpha or beta globin chains, resulting in reduced hemoglobin levels and impaired oxygen transport.
-
Carbon Monoxide Poisoning: Carbon monoxide (CO) binds to hemoglobin with much higher affinity than oxygen, forming carboxyhemoglobin. This prevents oxygen from binding, causing severe hypoxia and potentially death.
-
Methemoglobinemia: A condition where the iron in hemoglobin is oxidized to the ferric state (Fe³⁺), preventing oxygen binding.
Conclusion: The Crucial Role of Tetrameric Hemoglobin
The misconception that each hemoglobin molecule carries only two oxygen molecules is a significant error. The reality is that the tetrameric structure of hemoglobin, with its four heme groups and cooperative binding, enables the transport of four oxygen molecules per molecule. This efficient oxygen-carrying capacity is essential for life, and any disruption to this process has severe physiological consequences. Understanding the complexities of hemoglobin's function is vital for comprehending normal physiology and various pathological conditions affecting oxygen transport. Further research continues to unravel the intricacies of this remarkable protein and its pivotal role in maintaining life. Further investigations into the subtle nuances of oxygen binding and release are crucial for developing effective treatments for hemoglobin-related diseases and enhancing our understanding of respiratory physiology. The collaborative binding mechanism, influenced by pH, CO2 levels, 2,3-BPG, and temperature, ensures optimal oxygen delivery based on the body's changing metabolic demands, highlighting the intricate and elegant design of this essential protein. The efficient delivery of oxygen by hemoglobin is not merely a chemical reaction but a tightly regulated physiological process essential for maintaining cellular function and overall health.
Latest Posts
Related Post
Thank you for visiting our website which covers about Each Hemoglobin Molecule Can Transport Two Molecules Of Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.