650 J Is The Same Amount Of Energy As
New Snow
May 11, 2025 · 5 min read
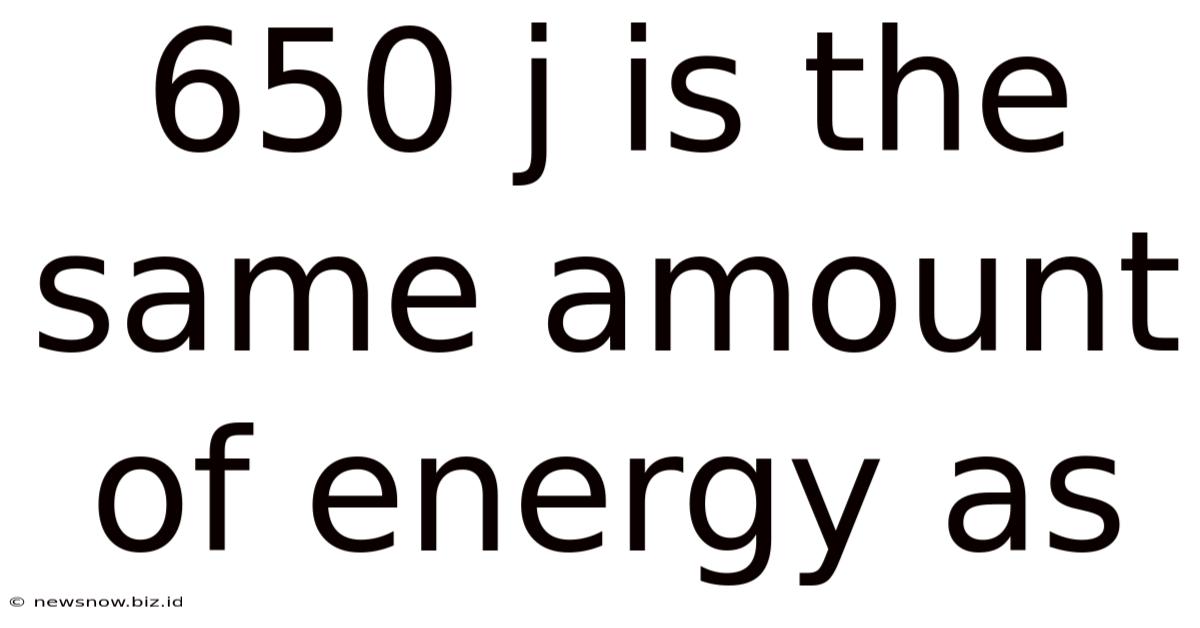
Table of Contents
650 J is the Same Amount of Energy As: Exploring Energy Equivalents and Conversions
Understanding energy is crucial in various fields, from physics and engineering to everyday life. Energy, in its many forms, is the capacity to do work. Often, we encounter energy measurements in Joules (J), a standard unit in the International System of Units (SI). But what does 650 J actually represent in tangible terms? This article explores the various equivalents of 650 Joules, illustrating its magnitude through relatable examples and clarifying the concepts of energy conversion and equivalence.
Understanding the Joule
Before diving into the equivalents of 650 J, let's solidify our understanding of the Joule itself. A Joule (J) is the SI unit of energy, work, and heat. It's defined as the amount of energy transferred to an object when a force of one newton acts on that object in the direction of its motion through a distance of one meter. In simpler terms, it's a measure of how much work can be done or how much energy is transferred.
It's crucial to remember that energy exists in various forms, including:
- Kinetic Energy: The energy of motion. A moving object possesses kinetic energy.
- Potential Energy: Stored energy due to an object's position or configuration. Gravitational potential energy is a common example (e.g., a book held above the ground).
- Thermal Energy: Energy associated with the temperature of an object or system.
- Chemical Energy: Energy stored in chemical bonds. This is released during chemical reactions.
- Electrical Energy: Energy carried by moving electric charges.
- Radiant Energy (Electromagnetic radiation): Energy that travels as waves, such as light and heat.
650 Joules: Real-World Equivalents
650 Joules isn't a massive amount of energy in the grand scheme of things. However, understanding its equivalents helps us grasp its magnitude in relation to everyday experiences. Let's explore some relatable examples:
Mechanical Work
-
Lifting a weight: 650 J is approximately the energy required to lift a 66-kilogram (around 145-pound) person a distance of one meter against the Earth's gravity (assuming 10 m/s² for gravitational acceleration). This is a surprisingly small amount of effort for a human.
-
Throwing a ball: The energy required to throw a baseball depends on its mass and velocity. A moderate throw could easily expend more than 650 J of energy. However, a slower, gentler throw would likely fall below this threshold. Calculating the precise speed for a 650 J throw requires considering the baseball's mass.
-
Pushing a cart: The energy expended in pushing a shopping cart a certain distance depends on the force applied and the distance covered. A relatively short push with moderate force could equate to 650 J.
Thermal Energy
-
Heating water: 650 J isn't enough to significantly heat a substantial volume of water. It might slightly warm a small amount, but the temperature change would be barely noticeable. The specific heat capacity of water plays a vital role in determining this temperature change.
-
Melting ice: Similarly, 650 J is insufficient to melt a considerable amount of ice. The latent heat of fusion for water (the energy required to change ice to water without a temperature change) is significantly higher. Only a small amount of ice would melt with this energy input.
Electrical Energy
-
Powering a small device: The energy consumption of small electronics, such as a low-power LED light or a small motor, can be in the range of Joules per second (Watts). A device using 650 J over a short period (e.g., a fraction of a second) is possible, depending on its power rating.
-
Charging a capacitor: A small capacitor could be charged to a certain voltage using 650 J of energy. The amount of charge stored and the capacitance value determine the energy stored.
Other Equivalents
-
Food Energy: The energy content of food is often expressed in kilocalories (kcal) or Calories (with a capital C). One kilocalorie is approximately 4184 J. Therefore, 650 J is a tiny fraction of a single Calorie—less than 0.2 Calories.
-
Chemical Reactions: Small chemical reactions, such as the burning of a tiny amount of fuel, could release energy in the order of hundreds of Joules. However, determining the exact quantity is complex, requiring detailed knowledge of the chemical reaction and stoichiometry.
Energy Conversion and Efficiency
A crucial concept to remember is energy conversion. Energy can change from one form to another, but the total energy remains constant (according to the law of conservation of energy). For example, the potential energy of water held behind a dam can be converted into kinetic energy as the water flows downhill, which can then be further converted into electrical energy in a hydroelectric power plant. However, real-world conversions are never 100% efficient; some energy is always lost as heat.
Understanding conversion efficiency is crucial when considering the equivalents of 650 J. If you're using 650 J of electrical energy to lift a weight, some energy will be lost as heat due to friction in the lifting mechanism. The actual work done on the weight will be less than 650 J.
Applications and Examples in Different Fields
The understanding of 650 Joules and its equivalents finds applications across numerous fields:
-
Physics: In mechanics, 650 J can be used to calculate the work done, kinetic energy, or potential energy of objects in motion or at rest.
-
Engineering: Engineers utilize Joules to design and analyze mechanical systems, power generation, and energy storage devices. Understanding energy efficiency is key to optimizing designs.
-
Medical Physics: In radiotherapy, the energy of radiation beams is measured in Joules, crucial for determining the appropriate dosage for cancer treatment.
-
Environmental Science: The energy content of various fuels and the energy balance of ecosystems are essential considerations in environmental studies.
Conclusion: Putting 650 Joules into Perspective
While 650 Joules may seem like a small number, it represents a measurable amount of energy with real-world applications. By considering its equivalents in mechanical work, thermal energy, electrical energy, and other forms, we can better understand its significance. Remember that energy conversion is always involved in practical applications, and efficiency plays a key role in determining the outcome. The ability to relate Joules to everyday phenomena is crucial for a deeper understanding of energy and its role in our world. This understanding helps us appreciate the energy transformations occurring constantly around us, from the simple act of lifting an object to complex industrial processes.
Latest Posts
Related Post
Thank you for visiting our website which covers about 650 J Is The Same Amount Of Energy As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.