1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
New Snow
May 11, 2025 · 6 min read
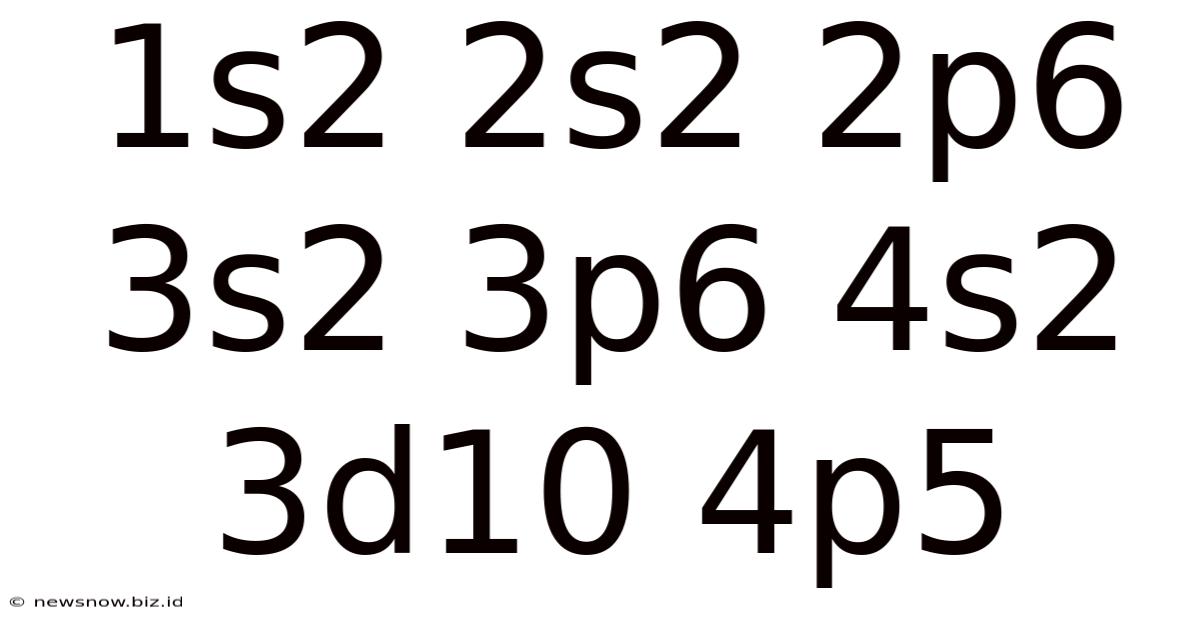
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵: Unveiling the Secrets of Bromine's Electron Configuration
The seemingly cryptic string of numbers and letters, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵, holds the key to understanding the fundamental properties of a fascinating element: bromine. This article delves deep into the meaning of this electron configuration, exploring its implications for bromine's atomic structure, chemical reactivity, and position within the periodic table. We will unravel the secrets behind this notation, explaining the underlying principles of electron filling and quantum numbers, and demonstrating how this knowledge allows us to predict bromine's behavior.
Understanding Electron Configurations: A Foundation in Atomic Structure
Before diving into the specifics of bromine's configuration, let's establish a solid understanding of what electron configurations represent. An electron configuration is a concise way of describing the arrangement of electrons within the electron shells and subshells of an atom. It's a fundamental concept in chemistry, providing insights into an atom's chemical properties and how it interacts with other atoms.
The Significance of Quantum Numbers
The electron configuration is governed by a set of quantum numbers that define the properties of atomic orbitals and the electrons that occupy them. These numbers are:
-
Principal Quantum Number (n): Represents the energy level or shell of an electron. It's a positive integer (1, 2, 3, etc.), with higher values indicating higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): Defines the shape of the orbital and its subshell. It can range from 0 to n-1. l=0 corresponds to an s orbital (spherical), l=1 to a p orbital (dumbbell-shaped), l=2 to a d orbital (more complex shapes), and l=3 to an f orbital (even more complex shapes).
-
Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space. It can take integer values from -l to +l, including 0. For example, a p subshell (l=1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can have a value of +½ or -½, representing "spin up" and "spin down," respectively. The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Deconstructing Bromine's Electron Configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Now, let's analyze bromine's electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Each part of this notation provides crucial information:
-
1s²: This indicates two electrons in the first energy level (n=1) occupying the s subshell (l=0). The s subshell can hold a maximum of two electrons.
-
2s²: Two electrons in the second energy level (n=2) occupying the s subshell (l=0).
-
2p⁶: Six electrons in the second energy level (n=2) occupying the p subshell (l=1). The p subshell consists of three orbitals, each capable of holding two electrons, hence a total of six electrons.
-
3s²: Two electrons in the third energy level (n=3) occupying the s subshell (l=0).
-
3p⁶: Six electrons in the third energy level (n=3) occupying the p subshell (l=1).
-
4s²: Two electrons in the fourth energy level (n=4) occupying the s subshell (l=0). Note that the 4s subshell fills before the 3d subshell, a consequence of the relative energies of these orbitals.
-
3d¹⁰: Ten electrons in the third energy level (n=3) occupying the d subshell (l=2). The d subshell has five orbitals, each holding two electrons, for a total of ten.
-
4p⁵: Five electrons in the fourth energy level (n=4) occupying the p subshell (l=1). This incomplete p subshell is responsible for many of bromine's chemical properties.
Bromine's Position in the Periodic Table and its Properties
Bromine's electron configuration directly relates to its position in the periodic table. It's a halogen, located in Group 17 (VIIA) and Period 4. The key feature here is the 4p⁵ configuration. Halogens are characterized by having seven valence electrons (electrons in the outermost energy level). This incomplete octet drives their high reactivity. Bromine readily gains an electron to achieve a stable octet configuration, forming the bromide ion (Br⁻). This explains why bromine is highly reactive and readily forms ionic compounds with metals.
Chemical Reactivity and Bonding
The presence of five electrons in the 4p subshell makes bromine a highly reactive nonmetal. Its strong electronegativity means it readily attracts electrons from other atoms, forming ionic or covalent bonds. Ionic bonds are formed with metals, where bromine accepts an electron to achieve a stable octet. Covalent bonds are formed with other nonmetals, where bromine shares electrons to achieve a stable electron configuration. This explains bromine's diverse range of compounds.
Physical Properties: A Consequence of Electron Configuration
Bromine's electron configuration also influences its physical properties. Bromine exists as a diatomic molecule (Br₂), a reddish-brown liquid at room temperature. The relatively weak intermolecular forces between Br₂ molecules contribute to its relatively low boiling point and volatility. The electron configuration explains these intermolecular interactions, illustrating the relationship between microscopic structure and macroscopic properties.
Applications of Bromine and its Compounds
The unique properties stemming from its electron configuration make bromine and its compounds incredibly useful across various applications:
-
Flame Retardants: Brominated flame retardants are extensively used in plastics, textiles, and electronics to reduce flammability. The bromine atoms interrupt the combustion process, hindering the spread of fire.
-
Agricultural Chemicals: Bromine compounds serve as fumigants and pesticides, although their use is becoming more restricted due to environmental concerns.
-
Water Purification: Bromine compounds are used as disinfectants in water treatment plants, similar to chlorine, to eliminate bacteria and other harmful microorganisms.
-
Pharmaceuticals: Bromine compounds find applications in several pharmaceuticals, contributing to the efficacy of certain drugs.
-
Photography: Bromide salts were historically used extensively in photography.
Environmental Considerations and Safety Precautions
While bromine and its compounds have extensive uses, their environmental impact and safety aspects cannot be overlooked. Certain brominated compounds are persistent organic pollutants (POPs), posing risks to human health and the environment. Proper handling and disposal of bromine compounds are essential to mitigate potential harm.
Conclusion: The Significance of Electron Configuration
The seemingly simple notation, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵, encapsulates the essence of bromine's atomic structure and dictates its chemical behavior. Understanding electron configurations is a cornerstone of chemistry, providing a framework for predicting the properties and reactivity of elements. Bromine's electron configuration, with its incomplete 4p subshell, explains its reactivity, its position in the periodic table as a halogen, and ultimately its numerous applications and associated environmental considerations. This detailed exploration showcases the powerful connection between the microscopic world of atomic structure and the macroscopic world of chemical properties and practical applications. The study of electron configurations is fundamental to mastering the intricacies of chemistry and unlocking a deeper understanding of the elements that make up our world.
Latest Posts
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.